

#Nitrate charge pdf#
Google Patents Method of charging water-filled blast holes with ammonium nitrate and primer cartridge used in sameĭownload PDF Info Publication number US2920523A US2920523A US663393A US66339357A US2920523A US 2920523 A US2920523 A US 2920523A US 663393 A US663393 A US 663393A US 66339357 A US66339357 A US 66339357A US 2920523 A US2920523 A US 2920523A Authority US United States Prior art keywords container ammonium nitrate water liquid oxygen explosive Prior art date Legal status (The legal status is an assumption and is not a legal conclusion. Google Patents US2920523A - Method of charging water-filled blast holes with ammonium nitrate and primer cartridge used in same Lakes that rely on ground water are often affected by nitrification through this process.US2920523A - Method of charging water-filled blast holes with ammonium nitrate and primer cartridge used in same Septics leach down into ground water resources or aquifers and supply near by bodies of water. Water quality may also be affected through ground water resources that have a high number of septic systems in a watershed. Specifically, they are a naturally occurring chemical that is left after the break down or decomposition of animal or human waste. Nitrates are also a by product of septic systems. Consequently, as nitrates form a component of total dissolved solids, they are widely used as an indicator of water quality. As well as leading to water anoxia, these blooms may cause other changes to ecosystem function, favouring some groups of organisms over others. These levels of nitrate can also lead to algae blooms, and when nutrients become limiting (such as potassium, phosphate or nitrate) then eutrophication can occur. In most cases of excess nitrate concentrations, the principle pathway of entering aquatic systems is through surface runoff from agricultural or landscaped areas which have received excess nitrate fertilizer. However, due to inherent problems with past protocols on acute nitrate toxicity experiments, nitrate may be less toxic to marine animals than previously thought. While nitrate is much less toxic than ammonia or nitrite, levels over 30 ppm of nitrate can inhibit growth, impair the immune system and cause stress in some aquatic species. In freshwater or estuarine systems close to land, nitrate can reach high levels that can potentially cause the death of fish.
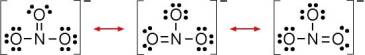
Organic compounds containing the nitro functional group (which has the same formula and structure as the nitrate ion save that one of the O − atoms is replaced by the R group) are known as nitro compounds. Nitrates should not be confused with nitrites (NO 2 −) the salts of nitrous acid. Examples are methyl nitrate formed by reaction of methanol and nitric acid, the nitrate of tartaric acid, and the inappropriately named nitroglycerin. They are the esters of nitric acid and alcohols formed by nitroxylation. In organic chemistry a nitrate is a functional group with general chemical formula RONO 2 where R stands for any organic residue. Almost all inorganic nitrate salts are soluble in water at standard temperature and pressure.


 0 kommentar(er)
0 kommentar(er)
